∞ generated and posted on 2016.08.18 ∞
Negative control of enzyme functioning that involves binding of a substance to an enzyme's active site.
The reason this is described as competitive inhibition is because normal substrate can literally compete for access to the active site with the inhibitor. In addition, substrate in sufficient concentrations can gain access to the active site to a greater extent than the inhibitor. Contrast, though, with allosteric inhibition, where the inhibitor does not bind to the active site and therefore cannot be competed off of the active site by the enzyme's normal substrate.
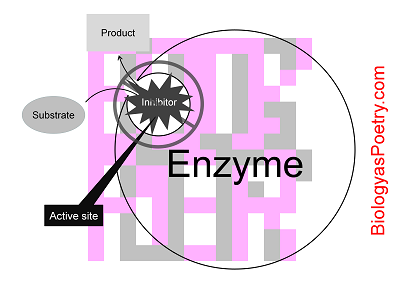
Figure legend: Note the attachment of the inhibitor molecule directly to the active site. The result is a modification of the active site in such a manner that the active site is no longer catalytically active. The interaction is reversible, however, resulting in a potential for normal substrate to compete with the competitive inhibitor for access to the active site, thereby temporarily bypassing inhibition.
Note that the reason that substrate can compete with competitive inhibitor for access to an enzyme's active site is because competitive inhibitors bind there reversibly. Thus, the more inhibitor that is present then the more likely that the inhibitor will be bound, but any particularly inhibitor molecule is constantly cycling between bound and not bound (with greater inhibitor affinity for the active site also resulting in greater likelihood of inhibitor being bound).
What the substrate does is literally wait until inhibitor is temporarily not present in an active site, with the more substrate present near the enzyme then the more likely that it will slip into the active site while the inhibitor is away. Indeed, to the degree that the substrate can block inhibitor binding then the more substrate that is present relative to inhibitor then the lower the potential that inhibitor may gain access to the active site.