∞ generated and posted on 2016.08.20 ∞
Long-chain carboxylic acid.
Long-chain refers specifically to long chains of carbon atoms making up hydrocarbons. At one end of this long hydrocarbon chain is a carboxyl group, an acidic functional group (as shown immediately below) and from which the name 'carboxylic acid' is derived.
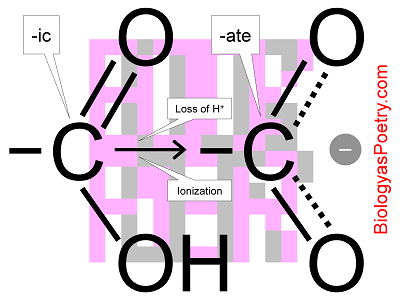
Figure legend: Ionization of a carboxyl group. To the left is the carboxyl group in its un-ionized form, bestowing on its carrier the suffix -ic (acid), as it in acetic acid (vinegar) or pyruvic acid (the three-carbon molecule produced by glycolysis). The loss of the hydrogen ion results in an averaging of bonds, a process known as resonance, which stabilizes the structure. So configured, the group is considered to be a salt rather than acid so is described using the suffix, -ate, as in acetate or pyruvate.
Fatty acids come in saturated, unsaturated, monounsaturated, polyunsaturated, and trans varieties. Combined chemically with glycerol they form fats and oils.
A carboxylic acid, more generally, is an organic molecule containing, as noted, a carboxyl group, which consists of the elements carbon, oxygen, and hydrogen, i.e., -COOH, and which can easily lose the H, forming a negatively charged ion (i.e., anion). Fatty acids thus are linear chains of carbon (C-C-C-C-C…) and associated hydrogens that have, at one end, a carboxylic acid group (e.g., C-C-C-C-C-COOH, plus associated hydrogen atoms).