∞ generated and posted on 2016.08.22 ∞
Chemical separation of subunits via addition of a water molecule, such as those subunits making up macromolecules.
That water molecule is split apart when these bonds are broken, with one part going to one subunit and the other to the other subunit. See by contrast, dehydration synthesis.
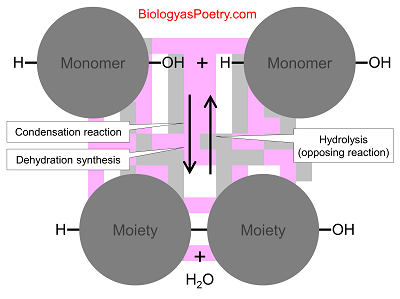
Figure legend: Shown is the opposing chemical reaction going from top to bottom (dehydration synthesis, a.k.a., condensation reaction). Hydrolysis instead is shown going from bottom to top. Note the insertion of the water molecule into the central bond between subunits, also known as moieties at this point, such as in amino acid moiety.
Generally hydrolysis is associated with catabolism rather than with anabolism. For example, the molecule ATP can be hydrolyzed to ADP and inorganic phosphate (Pi), liberating energy in the process. So too can disaccharides be hydrolyzed into their constituent monosaccharides or polypeptides into their amino acids, etc.