Highly hydrophilic, , and acidic component of nucleic acids as well as lipid bilayers.
Phosphate groups, also known simply as , supply the phosphate to AMP, ADP, and ATP. When a protein is it is a phosphate group that is being covalently attached. The phosophodiester linkages that connect together nucleotides in polynucleic acids also are phosphate groups.
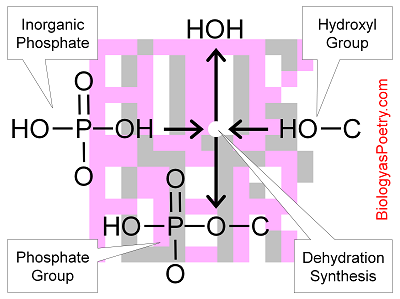
Generally, whenever you see or simply a "" in a biomolecule, it is a phosphate group that is present. Note that a phosphate that is found unattached to another atom or molecule is described as inorganic phosphate, which is abbreviated as Pi.
A phosphate group consists, basically, of , or PH3O4. Replace one of the hydrogens with another atom such as a carbon atom, or instead with an organic molecule, and the phosphoric acid becomes a phosphate group.
It is also possible to replace a second hydrogen, such as one sees with phosphodiester linkages. These replacements, though, often occur via a dehydration synthesis reaction such that, for example, a water molecule is removed from a combination of a hydroxyl group and the phosphate group to be. Like phosphoric acid, the phosphate group is prone to losing hydrogen ions and thus is both an acid and prone to taking on a negative charge.