∞ generated and posted on 2016.01.19 ∞
Overall solute concentrations lower on the outside than on the inside of cells; relatively speaking, dilute.
Contrast hypertonic and isotonic solutions. See also osmosis.
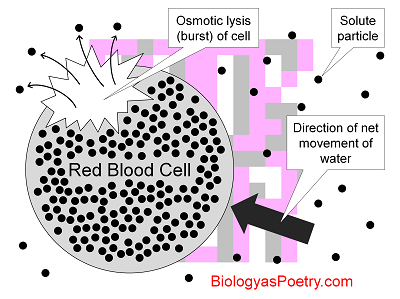
Figure legend: Red blood cells (RBCs), because they lack cell walls, are prone to osmotic lysis given suspension in a hypotonic, that is, low-solute solution. Because solute concentrations inside of these cells is high in comparison with outside, the water osmotically 'rushes' into the cells, expanding the cytoplasm to the point of bursting. Here the small, black circles found both inside and outside of the RBCs are solute particles, i.e., ions or molecules.
The following video shows nice experiments using dialysis tubing indicating the impact of osmosis on cell volume:
The following video is really nicely done, save for some math issues, using chicken eggs with dissolved shell as shrinking and expanding cells: