∞ generated and posted on 2016.08.22 ∞
The chemical connection between amino acids making up polypeptides.
Peptide bonds specifically are covalent linkages – chemical bonds in which electrons are shared among two atoms – that occur between one of the carbon atoms found in amino acids (the part that is associated with what is known as the carboxyl group) and the nitrogen atom that makes up the amino group.
More generally, the process forming a peptide bond is known as a condensation reaction or dehydration synthesis.
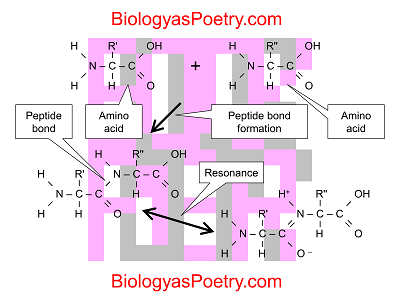
Figure legend: Condensation reaction between two amino acids forming a peptide bond. Not shown is the loss of a water molecule. Shown, however, are the resonance structures associated with the peptide bond. Note that double bond nature of the peptide bond seen in the resonance structure shown in the bottom right. The combination of the two structures results in a partial double bond nature of the peptide bond, resulting in greater stiffness to peptide chains than otherwise would be the case.
Due to something known as resonance, these chemical linkages take on what can be described as a partial double-bond character. That partial double-bond character in turn makes polypeptide chains much less rotatable than the single bonds that otherwise make up the "backbones" of polypeptides would suggest. The result is that polypeptide chains are stiffer than otherwise would be the case, which in turn results in a less "squishy" character to proteins.
Since proteins basically serve organisms as precise molecular machines, this greater structural rigidity is a helpful contributor to their ability to function in this capacity.